
2230
HEPATOLOGY, VOL. 68, NO. 6, 2018
Healthcare Cost and Utilization in
Nonalcoholic Fatty Liver Disease:
Real-World Data From a Large U.S.
Claims Database
Alina M. Allen,
1
Holly K. Van Houten,
2,3
Lindsey R. Sangaralingham,
2,3
Jayant A. Talwalkar,
1,2
and Rozalina G. McCoy
2,4,5
The prevalence of nonalcoholic fatty liver disease (NAFLD) is increasing. The health care burden resulting from the mul-
tidisciplinary management of this complex disease is unknown. We assessed the total health care cost and resource utiliza-
tion associated with a new NAFLD diagnosis, compared with controls with similar comorbidities. We used OptumLabs
Data Warehouse, a large national administrative claims database with longitudinal health data of over 100 million indi-
viduals enrolled in private and Medicare Advantage health plans. We identified 152,064 adults with a first claim for
NAFLD between 2010 and 2014, of which 108,420 were matched 1:1 by age, sex, metabolic comorbidities, length of fol-
low-up, year of diagnosis, race, geographic region, and insurance type to non-NAFLD contemporary controls from the
OptumLabs Data Warehouse database. Median follow-up time was 2.6 (range 1-6.5) years. The final study cohort con-
sisted of 216,840 people with median age 55 (range 18-86) years, 53% female, 78% white. The total annual cost of care per
NAFLD patient with private insurance was $7,804 (interquartile range [IQR] $3,068-$18,688) for a new diagnosis and
$3,789 (IQR $1,176-$10,539) for long-term management. These costs are significantly higher than the total annual costs
of $2,298 (IQR $681-$6,580) per matched control with similar metabolic comorbidities but without NAFLD. The largest
increases in health care utilization that may account for the increased costs in NAFLD compared with controls are repre-
sented by liver biopsies (relative risk [RR] = 55.00, 95% confidence interval [CI] 24.48-123.59), imaging (RR = 3.95, 95%
CI 3.77-4.15), and hospitalizations (RR = 1.87, 95% CI 1.73-2.02). Conclusion: The costs associated with the care for
NAFLD independent of its metabolic comorbidities are very high, especially at first diagnosis. Research efforts shouldfo-
cus on identification of underlying determinants of use, sources of excess cost, and development of cost-effective diagnostic
tests. (H 2018;68:2230-2238).
N
onalcoholic fatty liver disease (NAFLD) is
the most common chronic liver disease in
Western countries, affecting 24%
(1)
to 45%
(2)
of the U.S. population or 64-100 million people.
Most (approximately 80%) patients with NAFLD
have hepatic steatosis without inflammation, which
is associated with a relatively low risk of fibrosis,
(3,4)
but does have a strong correlation with cardiovascu-
lar disease, metabolic complications,
(5)
and increased
mortality compared with the general population.
(6)
The remaining 20% of patients have nonalcoholic
steatohepatitis (NASH), which can lead to cirrho-
sis, hepatocellular carcinoma, and other liver-related
complications.
(7)
Abbreviations: CI, confidence interval; ED, emergency department; ICD, International Classification of Diseases; IQR, interquartile range;
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OLDW, OptumLabs Data Warehouse; and RR, relative risk.
Received March 12, 2018; accepted May 8 2018.
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.30094/suppinfo.
A.M.A. is supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, American College of
Gastroenterology Junior Faculty Development Award, and the National Institute of Diabetes and Digestive and Kidney Diseases of the National
Institutes of Health (K23 DK115594). R.G.M. is supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health
Care Delivery and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K23 DK114497).
© 2018 by the American Association for the Study of Liver Diseases.
View this article online at wileyonlinelibrary.com.
DOI 10.1002/hep.30094
Potential conflict of interest: Nothing to report.

HEPATOLOGY, Vol. 68, No. 6, 2018 ALLEN ET AL.
2231
Given the increasing prevalence of NAFLD, the
economic burden is undoubtedly considerable, but real-
world data are scarce. U.S. health care expenditures have
steadily increased over the last decades and are pro-
jected to account for 20% of the economy by 2024.
(8,9)
The NAFLD epidemic wave could hasten this
increase; therefore, assessment of its contribution to
the economic burden and the major health care utiliza-
tion drivers is imperative. In a recent study, Younossi et
al. used Markov modeling to estimate the annual direct
healthcare costs at $1,612 per NAFLD patient.
(1)
However, as the authors acknowledged, the models
were constructed based on assumptions of NAFLD
epidemiology, fibrosis progression rate, and incident
complications, some of which were imputed from hep-
atitis C studies, resulting in uncertainty around many
inputs. Another study conducted among NAFLD
Medicare beneficiaries in 2010 estimated annual total
medical charges per patient to be $3,608 for outpa-
tient
(10)
and $36,289 for inpatient care.
(11)
Although
this provided direct cost data, it included an older pop-
ulation, whereas NAFLD is most prevalent in the mid-
dle age group (45-64 years).
Moreover, as the clinical care of NAFLD subjects is
directed not only by liver disease but also by the coex-
istent comorbidities, such as diabetes, hypertension or
cardiovascular disease, previous studies did not isolate
the specific contribution of NAFLD to the healthcare
burden from that of other metabolic diseases.
We therefore assessed the total health care cost and
use of patients with NAFLD, compared with a control
population with similar comorbidities, among commer-
cially insured and Medicare Advantage beneficiaries,
using a large administrative claims database. The data
set used in this study, OptumLabs Data Warehouse
(OLDW), is uniquely suited to study NAFLD burden
as it includes over 100 million people across the United
States, with greatest representation in the South, where
the prevalence of NAFLD is highest. It includes adults
of all ages, thereby updating and completing previously
published data that focused on Medicare beneficiaries.
The estimation of direct costs and utilization offers
better understanding about the financial implications
of NAFLD for patients and the health care system and
helps identify areas in need of better resource alloca-
tion, standardized management, and greater efficien-
cies in delivered care.
Methods
DATA SOURCE
This was a retrospective analysis of medical and
pharmacy claims data from the OLDW, a large national
administrative claims database that includes longitudinal
health data of more than 100 million individuals enrolled
in private and Medicare Advantage health plans since
1994,
(12,13)
which offers an excellent platform to trend
the cost of care
(14-16)
and private health insurance. The
population is diversely distributed in age, race, and geo-
graphical location in all 50 states. The database includes
deidentified enrollee information (sex, age, race/ethnic-
ity, region of residency, insurance plan), medical claims
(including diagnosis and procedure codes, site of service
codes, provider specialty codes, and total paid amounts),
and pharmacy claims. The study involved analysis of
preexisting de-identified data; thus, it was exempt from
Institutional Review Board approval.
STUDY POPULATION
We identified all patients with a first medical claim
for NAFLD using the International Classification of
Diseases (ICD) 9-CM 571.5 (cirrhosis of the liver
ARTICLE INFORMATION:
From the
1
Division of Gastroenterology and Hepatology, Department of Medicine, Mayo Clinic, Rochester, Minnesota;
2
Robert D.
and Patricia E. Kern Center for the Science of Health Care Delivery,Mayo Clinic, Rochester, Minnesota;
3
OptumLabs, Cambridge,
Massachusetts;
4
Division of Primary Care Internal Medicine, Department of Medicine,Mayo Clinic, Rochester, Minnesota;
5
Division of
Health Care Policy & Research, Department of Health Sciences Research,Mayo Clinic, Rochester, Minnesota.
ADDRESS CORRESPONDENCE AND REPRINT REQUESTS TO:
Alina M. Allen, M.D.
Division of Gastroenterology and Hepatology, Mayo Clinic
200 First Street SW
Rochester, MN 55905
E-mail: allen.alina@mayo.edu
Tel: +1-507-284 -3917
Fax: +1-507-284-0938
ALLEN ET AL. HEPATOLOGY, December 2018
2232
without mention of alcohol), 571.8 (other chronic non-
alcoholic liver disease), and 571.9 (unspecified chronic
liver disease without mention of alcohol) between
2010 and 2014. From this cohort, we excluded subjects
diagnosed with other liver diseases, including viral,
alcoholic, and cholestatic liver disease (ICD 9-CM
codes in Supporting Table S1). Subjects were clas-
sified as NAFLD cases if no alternative liver disease
was identified prior to the index NAFLD diagnosis or
during follow-up. This diagnostic algorithm correctly
identified true NAFLD cases with 85% accuracy in a
previously published retrospective population-based
cohort.
(5)
The service date of the first observed claim
for NAFLD was defined as the index date for patients
in the NAFLD cohort.
A control cohort was assembled by identifying
patients with at least one medical claim for an office
visit during 2010 to 2014 and no medical claims with
diagnosis codes for NAFLD or other liver diseases
during the study period. The controls were matched
1:1 on age, sex, race, diabetes mellitus, hypertension,
dyslipidemia, cardiovascular disease, length of fol-
low-up, year of diagnosis, geographic region, and insur-
ance type. The index date for the control cohort was
assigned to a randomly chosen office visit during the
identification period.
All subjects were continuously enrolled in the health
plan with medical and pharmacy benefits for at least 1
year before and 1 year after their index date. The sub-
jects were followed until disenrollment from the health
care plan or study end date ( June 2016). Supporting
Fig. S1 illustrates the study scheme.
COVARIATES AND OUTCOMES OF
INTEREST
Comorbidities associated with NAFLD including
diabetes mellitus, hypertension, dyslipidemia, and car-
diovascular disease were identified using the diagnos-
tic codes listed in Supporting Table S2. The NAFLD
subjects and controls were matched on these comor-
bidities at the index date to maximize the association
of cost and utilization with NAFLD and not with its
comorbidities. Outcomes of interest were direct costs
and health care use, such as office visits, hospitaliza-
tions, emergency department (ED) visits, as well as
tests and procedures attributable to liver disease: liver
biopsy, imaging (ultrasound, abdominal computed
tomography, and magnetic resonance imaging), and
laboratory tests (Supporting Tables S3 and S4). The
outcomes were measured at three different time points
in reference to the index date of NAFLD diagnosis or
matching: 1 year before, 1 and 5 years after.
STATISTICAL ANALYSIS
Patient characteristics (age, sex, race, census region,
year of diagnosis, comorbidities, and insurance type)
were described using mean (SD) or count (percentage)
as appropriate. Unadjusted utilization rates and total
costs of care were compared between NAFLD cases
and controls for 1 year prior to diagnosis date, 1 year
following diagnosis date, and 5 years following diagno-
sis date. Total cost of care included both medical (inpa-
tient and outpatient) claims and outpatient pharmacy
claims. Total health care costs were reported per patient
and were inflation-adjusted to 2015 U.S. dollars using
the Consumer Price Index.
(17)
Health care resource
use was identified as rates (number of events per 1000
patients) and rate ratios between 1 year post-index
date versus 1 year pre-index date and NAFLD versus
controls. Data were analyzed separately for privately
insured and Medicare Advantage subjects. Statistical
analyses were performed in SAS version 9.4 (SAS
Institute, Cary, NC).
Results
We identified 350,406 people with a first diagnosis
of NAFLD between 2010 and 2014, of which 165,281
were excluded for lack of medical and pharmacy coverage
at least 1 year prior to and 1 year after the index NAFLD
diagnosis. Additionally, 33,061 people were excluded
due to concurrent liver diseases other than NAFLD.
From the remaining cohort of 152,064 people with inci-
dent NAFLD, 108,420 were matched 1:1 by age, sex,
metabolic comorbidities, length of follow-up, year of
diagnosis, race, geographic region, and insurance type to
non-NAFLD contemporary controls from the OLDW
database. We were unable to match all NAFLD patients
to controls due to the multitude of matching variables.
The final study cohort consisted of 216,840 people with
median age 55 (range 18-86) years, 53% female and 78%
white (Table 1). Median follow-up time was 2.6 (range
1-6.5) years for both NAFLD and controls.
HEALTHCARE COSTS IN NAFLD
Figure 1 shows the annual total health care costs
of NAFLD subjects compared with matched con-
trols, in reference to the date of index (first) diagnosis

HEPATOLOGY, Vol. 68, No. 6, 2018 ALLEN ET AL.
2233
or matching, respectively. We show the total annual
costs starting 1 year prior to the index date, to allow
comparisons within the peridiagnosis period (1 year
before versus 1 year post), as well as long-term annual
costs,reflective of disease monitoring and management
of comorbidities. For both NAFLD subjects and con-
trols, the costs of care for Medicare Advantage enroll-
ees were considerably higher than for subjects with
private insurance.
The costs were highest during the first year follow-
ing the index NAFLD diagnosis, likely reflecting the
costs of diagnosis and initial evaluation for NAFLD
and its comorbidities. Specifically, among patients with
commercial insurance the median cost of medical care
during the year following NAFLD diagnosis increased
by 72%, from $4,547 (interquartile range [IQR]
$1,648-$11,661) to $7,804 (IQR $3,068-$18,688).
The median costs for Medicare Advantage enrollees
with NAFLD increased by 38%, from $6,566 (IQR
$3139-$14,787) during the year prior to NAFLD
diagnosis to $9,062 (IQR $4,313-$20,765) during the
year after diagnosis. For reference, the annual health-
care costs of non-NAFLD matched controls increased
only by 5% to 10% after the index date, in line with the
expected increase in annual rates.
The annual health care costs in the subsequent years
were lower than the immediate peri-diagnosis period.
Nevertheless, the annual costs for NAFLD patients
remained considerably higher than those for matched
controls. Specifically, at 5 years following NAFLD
diagnosis, the median annual health care cost was
$3,789 (IQR $1,176-$10,539) per NAFLD patient
with commercial insurance and $2,298 (IQR $681-
$6,580) per control. Among the Medicare Advantage
population, the median annual health care cost was
$5,363 (IQR $2,402-$12,515) per NAFLD patient
and $4,111 (IQR $1,677-$9,958) per control.
Consequently, the median cumulative healthcare
costs 5 years following the index NAFLD diagnosis
TABLE 1. Characteristics of NAFLD Patients and Matched
Controls
Controls
n = 108,420
NAFLD
n = 108,420
Age (years)
Median (IQR) 55 (45-65) 55 (45-65)
Age groups (years)
18-34 9341 (8.6%) 9341 (8.6%)
35-54 43,599 (40.2%) 43,599 (40.2%)
55-64 28,147 (26.0%) 28,147 (26.0%)
≥65 27,333 (25.2%) 27,333 (25.2%)
Gender
Female 57,167 (52.7 %) 57,167 (52.7 %)
Male 51,253 (47.3%) 51,253 (47.3%)
Index year
2010 19,663 (18.1%) 19,663 (18.1%)
2011 19,890 (18.3%) 19,890 (18.3%)
2012 22,538 (20.8%) 22,538 (20.8%)
2013 22,111 (20.4%) 22,111 ( 20.4%)
2014 24,218 (22.3%) 24,218 (22.3%)
Region
Midwest 27,230 (25.1%) 27,230 (25.1%)
Northeast 12, 8 26 (11.8%) 12,826 (11.8 %)
South 55,269 (51.0%) 55,269 (51.0%)
West 13,095 (12.1%) 13,095 (12.1%)
Race
White 84,613 (78.0%) 84,613 (78.0%)
Asian 2742 (2.5%) 2742 (2.5%)
Black 8508 (7.8%) 8508 (7.8%)
Hispanic 11,051 (10.2%) 11,051 (10.2%)
Unknown 1506 (1.4%) 1506 (1.4%)
Comorbidities
Hypertension 66,064 (60.9%) 66,064 (60.9%)
Hyperlipidemia 69,549 (64.1%) 69,549 (64.1%)
Cardiovascular disease 33,418 (30.8%) 33,418 (30.8%)
Diabetes mellitus 30,906 (28.5%) 30,906 (28.5%)
Insurance type
Commercial 76,697 (70.7%) 76,697 (70.7%)
Medicare Advantage 31,723 (29.3%) 31,723 (29.3%)
FIG. 1. Annual total health care costs of NAFLD patients
compared with matched controls in reference to the date of
index (first) NAFLD diagnosis or matching, respectively.
Abbreviations: NAFLD MA, NAFLD patients with Medicare
Advantage; NAFLD commercial, NAFLD patients with
commercial insurance; controls MA, matched controls with
Medicare Advantage; controls commercial, matched controls
with commercial insurance.
ALLEN ET AL. HEPATOLOGY, December 2018
2234
for an individual with commercial insurance were
nearly 80% higher than a control with similar age
and comorbidities: $30,994 (IQR $14,688- $64,972)
versus $17,345 (IQR $7,198-$38,713). The median
cumulative 5-year costs for a NAFLD individual with
Medicare Advantage were 42% higher than controls:
$39,588 (IQR $20,950-$71,226) versus $27,777 (IQR
$14,192-$54,666).
HEALTH CARE UTILIZATION IN
NAFLD
To explore what health care utilization parameters
may account for higher cost of care in NAFLD, we
assessed several utilization indices at similar time-
frames used for the cost estimates: peri-diagnosis and
at 5 years after the index diagnosis. In reference to the
year prior to the index date, most utilization parame-
ters during the following year increased slightly among
controls, as expected with the passage of time and aging,
but the rise was markedly higher among patients newly
diagnosed with NAFLD. The largest increase in use
(rate per 1000 patients) after NAFLD diagnosis was
liver biopsy from 5.5 to 28.8, followed by liver-related
imaging and all-cause hospitalizations. There were
smaller, but consistent, increases in laboratory testing
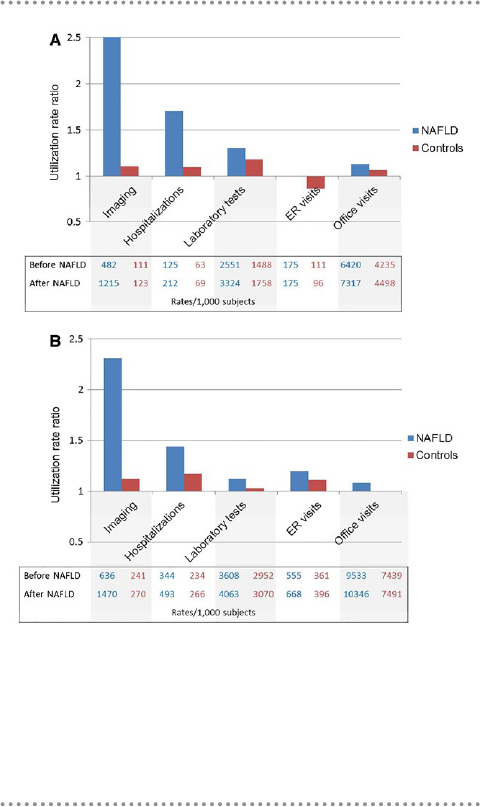
episodes, ED visits, and office visits. Figure 2A demon-
strates the relative change in utilization rates among
commercially insured patients with NAFLD when
compared with controls. Patients with NAFLD expe-
rienced substantial increases in use of imaging (relative
risk [RR] = 2.52, 95% confidence interval [CI] 2.49-
2.56), hospitalizations (RR = 1.69, 95% CI 1.64-1.75),
and laboratory tests (RR = 1.30, 95% CI 1.29-1.32)
when compared with controls, in whom the relative
increases were minimal. Among the most commonly
used imaging modalities, magnetic resonance imag-
ing showed the highest increase after diagnosis (RR =
3.42, 95% CI 3.20-3.66), followed by ultrasound (RR
= 2.77, 95% CI 2.71-2.82) and computed tomography
(RR = 2.57, 95% CI 2.52-2.62) (Supporting Table S5).
The trends were similar among the 63,442 subjects
with Medicare Advantage insurance, in whom the
largest increases in utilization after NAFLD diagnosis
were due to increased rates of liver biopsy, imaging, and
hospitalizations (Fig. 2B and Supporting Table S5).
Longitudinal follow-up data at 5 years following
NAFLD diagnosis/matching were available in a subset
of 20,840 individuals. The cumulative healthcare uti-
lization remained significantly higher among patients
with NAFLD compared with controls (Table 2).
Among commercially insured beneficiaries, liver biop-
sies continued to account for the largest difference in use
in NAFLD compared with controls (RR = 55.00, 95%
CI 24.48-123.59), followed by imaging (RR = 3.95,
95% CI 3.77-4.15) and hospitalizations (RR = 1.87,
95% CI 1.73-2.02). Among the Medicare Advantage
beneficiaries, the largest differences in health care use
with NAFLD were due to liver biopsies, imaging, and
ED visits.
The average cumulative rate of overall outpatient
office visits at 5 years following diagnosis was 40%
higher among patients with NAFLD compared with
controls: 31,079 versus 22,244 visits per 1000 patients.
Only 4.6% of these visits were to gastroenterology spe-
cialists, whereas 46% were to primary care (Fig. 3). The
proportion of other specialty visits, such as endocrinol-
ogy and cardiovascular diseases, was similar between
NAFLD and controls (2.4% and 3%, respectively),
reflective of robust matching by comorbidity status
FIG. 2. Relative change in utilization rates after a new diagnosis
of NAFLD compared with matched controls. (A) Commercial
insurance enrollees. (B) Medicare Advantage enrollees. The
bars represent utilization rate ratios (rates 1 year after diagnosis/
matching/rates 1 year prior to diagnosis/matching). The
corresponding absolute rates are presented below the bars.

HEPATOLOGY, Vol. 68, No. 6, 2018 ALLEN ET AL.
2235
during cohort selection. In the Medicare Advantage
cohort, gastroenterology visits represented 3.0% of all
visits among patients with NAFLD compared with
1.6% of all visits among controls.
Discussion
Using real-world data from a large nationwide
medical claims database, we show that the long-term
cumulative health care cost of a NAFLD patient is
80% higher than that of a non-NAFLD control of
similar age and metabolic comorbidities. The highest
annual costs occur around a new diagnosis of NAFLD,
reaching $7,804 and $9,062 per individual with pri-
vate insurance and Medicare Advantage, respectively.
Annual costs for long-term management decrease
to $3,789 and $5,363 per individual with private
insurance and Medicare Advantage, respectively, but
remain considerably higher than controls. The largest
increases in health care utilization, which may account
for the increased costs in NAFLD, are represented
by liver biopsies, imaging, and hospitalizations. The
large burden of NAFLD is managed predominantly
by primary care physicians, whereas subspecialty vis-
its in gastroenterology represent only 3 to 4.6% of
the total office visits. These data highlight that, as the
NAFLD burden will continue to increase,
(18)
solutions
are needed to promote innovative health care delivery
TABLE 2. Cumulative Use Rates per 1000 Patients at 5 Years After NAFLD Diagnosis
Controls NAFLD Rate Ratio (95% CI)
A. Commercial insurance
n 7464 7464
Liver biopsy 0.8 44.2 55.00 (24.48, 123.59)
Imaging 567.3 2243.2 3.95 (3.77, 4.15)
Ultrasound 144.0 762.7 5.30 (4.95, 5.66)
Computer tomography 287.5 1085.6 3.78 (3.54, 4.03)
Magnetic resonance imaging 19.0 116.0 6.10 (4.94, 7.53)
Transient elastography 0.0 0.4 —
Laboratory tests 8,517.0 12,380.5 1.45 (1.41, 1.49)
Hospitalizations 263.1 492.4 1.87 (1.73, 2.02)
Outpatient visits 22,243.7 31,078.8 1.40 (1.36, 1.43)
ED visits 328.5 402.7 1.23 (1.16, 1.30)
B. Medicare Advantage
n 2956 2956
Liver biopsy 1.0 25.0 24.67 (7.77, 78.34)
Imaging 1167.1 3297.0 2.82 (2.64, 3.02)
Ultrasound 182.3 879.2 4.82 (4.36, 5.33)
Computer tomography 651.6 1758.4 2.70 (2.49. 2.92)
Magnetic resonance imaging 36.2 126.5 3.50 (2.69, 4.55)
Transient elastography 0.0 0.0 -
Laboratory tests 14,740.2 17,169. 5 1.16 (1.10, 1.23)
Hospitalizations 1041.6 1489.5 1.43 (1.32, 1.54)
Outpatient visits 35,903.9 45,885.0 1.28 (1.23, 1.32)
ED visits 1724.0 2732.1 1.58 (1.48, 1.70)
FIG. 3. Average cumulative rate of overall outpatient office visits
5 years after diagnosis/matching and distribution by medical
specialties of interest.
ALLEN ET AL. HEPATOLOGY, December 2018
2236
platforms to reduce costs and to provide primary care
physicians with the necessary strategies and resources
to optimally manage this complex patient population.
The disease characteristics and the enormous clin-
ical burden of NAFLD pose considerable challenges
to the medical community, which extend beyond the
hepatology field. In this cohort, a strikingly low pro-
portion of the outpatient visits were represented by
gastroenterology and hepatology. The overwhelming
clinical burden of NAFLD is supported by general
practitioners, who have a key role in the identification,
risk stratification, and timely referral for specialty care
in NAFLD, butmay be unfamiliar with the intricacies
of the disease.
(19)
The American Association for the
Study of Liver Diseases guidelines suggest vigilance
for NAFLD, but do not provide well-defined screen-
ing recommendations for primary care providers and
cost-effective methods of disease severity assess-
ment.
(20)
The lack of clear guidelines is due to uncer-
tainties surrounding cost-effectiveness of diagnostic
tests and long-term benefits of screening, which are
areas in significant need of further research in the
hepatology community. The current state of NAFLD
diagnosis and disease severity assessment is based on
combinations of several available tests that include lab-
oratory studies, ultrasound, cross-sectional imaging,
elastography and liver biopsy, the use of which is sub-
ject to individual practice patterns.
Although the most cost-effective modality to esti-
mate disease severity in NAFLD remains to be estab-
lished,
(21)
these data offer a much-needed synopsis of
the real-world practice. The total costs soar by 72% in
the first year after the initial NAFLD claim and reach
exorbitant levels when compared with non-NAFLD
controls. Increases in utilization corresponding to
these costs were noted among all diagnostic modalities,
but were dominated by imaging tests (with costs that
vary between $200 and $3000
(22)
), which increased
2.5-fold (1215 per 1000 patients). It has been recog-
nized that using ultrasound to detect hepatic steatosis
is not cost-effective because clinically relevant fibro-
sis is present in no more than 11% of cases.
(23,24)
The
utility of other modalities, including elastography,
which is potentially more effective but costlier, has not
yet been proven. Although liver biopsy is required to
diagnose NASH, only patients at high risk require this
evaluation. In this cohort, liver biopsy, with a cost that
generally varies between $1500 and $3000
(22)
had the
highest relative increase in use (5-fold), although the
absolute rate of utilization remained low (29 per 1000
patients). The use of labs for diagnosis (the least expen-
sive but also least reliable alternative) increased by 30%.
The exorbitant costs of care around the first diagnosis
of NAFLD in this cohort underline the acute need of
more cost-effective methods of screening and disease
severity assessment.
The annual health care costs for NAFLD remained
extremely high beyond the initial peri-diagnosis period.
The long-term annual costs of NAFLD management
are almost double those of the matched cohort ($3789
versus $2298 per subject). Over the 5 years following
the index diagnosis, NAFLD patients are subjected
to abdominal imaging 4-fold more frequently than
matched controls. Similarly, the rate of blood testing
and outpatient visits is 45% and 39% higher, respec-
tively. The relative cost difference between NAFLD
and control patients was higher among the commer-
cially insured (younger) population than it was among
Medicare Advantage enrollees (in which cost is largely
driven by multimorbidity), suggesting that diagnosis
of NAFLD at earlier age in the context of increas-
ing NAFLD incidence in children and young adults
leads to a higher-cost differential at initial diagnosis.
Moreover, diagnosis at an earlier age leads to longer
follow-up time and monitoring for fibrosis progression
or surveillance for hepatocellular carcinoma. These
data highlight the need for cost-effective measures
to identify patients at high risk of disease progression
(i.e., differentiating patients with NASH and/or fibro-
sis from simple steatosis).
Although robust direct comparisons of long-term
costs in other liver diseases are not available, inferences
from hepatitis C models estimating $90,127 life-time
cost per patient treated with direct acting antiviral
agents
(25)
allow estimations that the cost of NAFLD
care is likely to surpass that of hepatitis C, especially in
light of upcoming NASH therapies.
These data are an essential benchmark for future
cost analyses in NAFLD, as several findings cover
important gaps in the existing literature. First, direct
costs are estimated from a large, nationally representa-
tive medical claims database. The annual direct cost per
NAFLD patient is approximately 5-fold higher than
previous estimates from the United States ($1612.18)
and European countries (€354-€1163) that relied on
Medicare data or derived from statistical modeling.
(1,11)
This is in part due to our ability to capture costs for
commercially insured adults who have heretofore been
excluded from NAFLD studies, despite being most of
the patients affected by the disease. Second, by using

HEPATOLOGY, Vol. 68, No. 6, 2018 ALLEN ET AL.
2237
a matched cohort with similar metabolic comorbidi-
ties as reference, we can differentiate liver-related costs
from those related to metabolic complications. Third,
we evaluate the costs at multiple time points and show
that the costs vary in reference to a new diagnosis.
Finally, we identify health care utilization in NAFLD
management, which is an important benchmark for
future cost-effectiveness analyses.
However, patients with Medicaid health coverage,
the uninsured, or those with NAFLD that remains
undiagnosed are not captured in OLDW; thus, prev-
alence estimates should not be extrapolated from this
study. Similarly, societal costs, derived from absentee-
ism and caregiver burden, certainly add even further
to the overall health care burden of NAFLD. As an
inherent limitation of large claims databases, we did
not have the opportunity to distinguish among clini-
cally appropriate and redundant use of tests, impact on
patient outcomes, and sources of excess costs. Further
work is needed to identify underlying determinants of
use, how to avoid high use of low-value services, and
insufficient use of high-value services that can drive
inefficient allocation of resources.
(26)
The care of NAFLD patients is expensive. As diag-
nostic methods and therapies for NAFLD become
increasingly available, early detection of the millions of
patients in the primary care setting, adequate risk strat-
ification, subspecialty referral and monitoring, while
taking into account cost-effectiveness, remains an
enormous challenge. Research efforts should focus on
development of high-value diagnostic tests to monitor
for liver fibrosis progression at appropriate intervals, in
a selected at-risk population, with the ultimate goal to
improve quality of care for the individual patient, while
being mindful of the effects on health care use.
REFERENCES
1) Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M,
Younossi Y, et al. The economic and clinical burden of non-
alcoholic fatty liver disease in the United States and Europe.
H 2016;64:1577-1586.
2) Williams CD, Stengel J, Asike MI, Torres DM, Shaw J,
Contreras M, et al. Prevalence of nonalcoholic fatty liver disease
and nonalcoholic steatohepatitis among a largely middle-aged
population utilizing ultrasound and liver biopsy: a prospective
study. Gastroenterology 2011;140:124-131.
3) Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba
R. Fibrosis progression in nonalcoholic fatty liver vs nonal-
coholic steatohepatitis: a systematic review and meta-analysis
of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:
643-654.
4) Rinella ME. Nonalcoholic fatty liver disease: a systematic re-
view. JAMA 2015;313:2263-2273.
5) Allen AM, Terry TM, Larson JJ, Coward A, Somers VK,
Kamath PS. Nonalcoholic fatty liver disease incidence and im-
pact on metabolic burden and death: a 20 year-community study.
H 2018;67:1726-1736.
6) Adams LA, Lymp JF, St. Sauver J, Sanderson SO, Lindor KD,
Feldstein A, et al. The natural history of nonalcoholic fatty liver
disease: a population-based cohort study. Gastroenterology
2005;129:113-121.
7) Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu
YC, McCullough AJ. Nonalcoholic fatty liver disease: a spec-
trum of clinical and pathological severity. Gastroenterology
1999;116:1413-1419.
8) Fuchs VR. How and why US health care differs from that in
other OECD countries. JAMA 2013;309:33-34.
9) Hartman M, Martin AB, Espinosa N, Catlin A, The National
Health Expenditure Accounts Team. National health care spend-
ing in 2016: spending and enrollment growth slow after initial
coverage expansions. Health Aff (Millwood) 2018;37:150-160.
10) Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C,
Mishra A. Trends in outpatient resource utilizations and out-
comes for Medicare beneficiaries with nonalcoholic fatty liver
disease. J Clin Gastroenterol 2015;49:222-227.
11) Sayiner M, Otgonsuren M, Cable R, Younossi I, Afendy M,
Golabi P, et al. Variables associated with inpatient and outpa-
tient resource utilization among medicare beneficiaries with
nonalcoholic fatty liver disease with or without cirrhosis. J Clin
Gastroenterol 2017;51:254-260.
12) Optum. https://www.optumlabs.com/. Accessed February 6, 2018.
13) Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH.
Optum Labs: building a novel node in the learning health care
system. Health Aff (Millwood) 2014;33:1187-1194.
14) Thayer S, Bell C, McDonald CM. The direct cost of manag-
ing a rare disease: assessing medical and pharmacy costs asso-
ciated with Duchenne muscular dystrophy in the United States.
J Manag Care Spec Pharm 2017;23:633-641.
15) Brady BL, Tkacz J, Meyer R, Bolge SC, Ruetsch C. Assessment
of rheumatoid arthritis quality process measures and associated
costs. Popul Health Manag 2017;20:31-40.
16) Weaver J, Sajjan S, Lewiecki EM, Harris ST, Marvos P.
Prevalence and cost of subsequent fractures among U.S. pa-
tients with an incident fracture. J Manag Care Spec Pharm
2017;23:461-471.
17) Department of Labor Bureau of Labor Statistics. Consumer
Price Index: chained consumer price index for all urban consum-
ers (C-CPI-U). Washington, DC; 2017.
18) Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling
the epidemic of nonalcoholic fatty liver disease demonstrates
an exponential increase in burden of disease. H
2018;67:123-133.
19) Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of
nonalcoholic steatohepatitis and associated practice patterns
of primary care physicians and specialists. BMC Res Notes
2016;9:157.
20) Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella
M, et al. The diagnosis and management of nonalcoholic fatty
liver disease: practice guidance from the American Association
for the Study of Liver Diseases. H 2018;67:328-357.
21) Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N.
Cost-effectiveness analysis: risk stratification of nonalcoholic
fatty liver disease (NAFLD) by the primary care physician using
the NAFLD fibrosis score. PLoS One 2016;11:e0147237.

ALLEN ET AL. HEPATOLOGY, December 2018
2238
22) Costhelper. http://health.costhelper.com. Accessed January 9,
2018.
23) Wright AP, Desai AP, Bajpai S, King LY, Sahani DV, Corey
KE. Gaps in recognition and evaluation of incidentally identified
hepatic steatosis. Dig Dis Sci 2015;60:333-338.
24) Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish
Murad S, Taimr P, et al. Presence of diabetes mellitus and ste-
atosis is associated with liver stiffness in a general population: the
Rotterdam study. H 2016;63:138-147.
25) Younossi ZM, Park H, Saab S, Ahmed A, Dieterich D, Gordon
SC. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens
in patients with chronic hepatitis C virus genotype 1 infection.
Aliment Pharmacol Ther 2015;41:544-563.
26) Elshaug AG, Rosenthal MB, Lavis JN, Brownlee S, Schmidt H,
Nagpal S, et al. Levers for addressing medical underuse and over-
use: achieving high-value health care. Lancet 2017;390:191-202.
Supporting Information
Additional Supporting Information may be found at
onlinelibrary.wiley.com/doi/10.1002/hep.30094/suppinfo.
