
2018
AP Chemistry
Sample Student Responses
and Scoring Commentary
Inside:
Free Response Question 6
• Scoring Guideline
• Student Samples
• Scoring Commentary
© 2018 The College Board. College Board, Advanced Placement Program, AP, AP Central, and the acorn logo
are registered trademarks of the College Board. Visit the College Board on the Web: www.collegeboard.org.
AP Central is the ocial online home for the AP Program: apcentral.collegeboard.org

201
8
SCORING GUIDELINES
Question 6
A student sets up a galvanic cell at 298 K that has an electrode
of
Ag(s) immersed in a 1.0 M solution
of
Ag+(aq) and an electrode
of
Cr(s) immersed
in
a 1.0 M solution
of
cr3+(aq), as shown in the diagram above.
(a) The student measures the voltage of the cell shown above and discovers that it
is
zero. Identify the
missing component
of
the cell, and explain its importance for obtaining a nonzero voltage.
The salt bridge is missing. The salt bridge allows for the
migration
of
ions to maintain charge balance in each half-cell.
1 point
is
earned for the correct
answer and a valid explanation.
Half-Reaction
E
(V)
Ag+(aq) +
e
Ag(s)
+0.80
Cr
3
+(aq) + 3
e
Cr(s)
?
(b) The student adds the missing component to the cell and measures
E
0
cell
to
be+
1.54 V. As the cell
operates,
Ag+
ions are reduced. Use this information and the information in the table above to do the
following.
(i) Calculate the value
of
E
for the half-reaction
Cr
3
+(aq) + 3 e
Cr(s).
0
E
cell
=
EE
red
(cathode) -
red
(anode)
+1.54 v = +0.80 v
x
x
= +0.80 V
(+1.54
V)
=
0.74 V
1 point
is
earned for a correct calculation
of
E
0
red
(ii) Write the balanced net-ionic equation for the overall reaction that occurs as the cell operates.
3
Ag+(aq) + Cr(s)
3 Ag(s) + cr3+(aq)
1 point
is
earned for the correctly balanced equation.
AP
®
CH
EMISTRY
© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

(iii) Calculate the value
of
G
for the overall cell reaction in J/molrxn.
æö
G:)
=
3
mole
-
æ
c J
D --
nFE
=
ç
96 485
(
1 54
ç÷
è
1 molwz
ø
è
mole
-
c
)
=
4.46
I 0
5
J/molrxn
I point
is
earned for the correct
calculation
of
the value of
G.
AP
®
CHEMISTRY
2018 SCORING GUIDELINES
© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.
Question 6 (continued)
ö
÷
ø

Ag(s)
M
i.o
?
0
.
A ,
© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

1·
-
.
-
© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

M
(V
)
X
OAlV,/
]· t
\1,ot >' of
0
c,'
Ctu
DY · h -7&
'
r
. - 0
© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.
in
?
•,
0
\
D.
GO ON TO THE NEXT PAGE.
© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

0
:
.•
'
\
'
'
© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.
AP
®
CHEMISTRY
2018 SCORING COMMENTARY
Question 6
Overview
This question required students to identify that the salt bridge was missing and to articulate its role in a standard
galvanic cell. They then needed to calculate the standard reduction potential of the anode, given the cathode
potential and the overall cell potential. Students were asked to write the balanced net-ionic equation for the
overall reaction and then to calculate the standard Gibbs free energy change for the overall cell reaction.
In part (a) a schematic drawing of an electrochemical cell was provided, with the salt bridge omitted. The
question asked students to identify the missing component of the cell and to explain its importance for obtaining
a nonzero voltage (LO 3.12; SP 2.2, 2.3, 6.4). A correct response to this question required identification of the
missing salt bridge and a discussion of its role in allowing for the migration of ions between half-cell
compartments. This component is necessary to maintain charge balance during the operation of the cell.
In part (b)(i) students were asked to calculate the value of E for the standard reduction of
3+
Cr (LO 3.13; SP 5.1).
The question indicated that
+
Ag ions are reduced, so students needed to deduce that chromium is oxidized in the
overall cell reaction. In part (b)(ii) the question required the chromium and silver half-reactions from the data
table to be combined into a balanced chemical equation appropriate for a galvanic cell (LO 3.2; SP 1.5, 7.1).
Finally, in part (b)(iii), the students were asked to calculate the standard Gibbs free energy change for the overall
cell reaction (LO 5.14; SP 2.2).
Sample: 6A
Score: 4
In part (a) the student states that the salt bridge “allows cations to flow to the cathode and anions flow to the
anode” and discusses how this maintains “electric neutrality.” These statements cover the basic elements of
“migration of ions” and maintaining “charge balance” expressed in the rubric; thus, 1 point was earned. In part
(b)(i) the student correctly calculates the value of E
red
for the chromium half-cell reaction and earned 1 point. In
part (b)(ii) the student correctly balances the equation, and 1 point was earned. In part (b)(iii) the student correctly
calculates the value of G and
earned 1 point.
Sample: 6B
Score: 2
In part (a) the student does not address the migration of ions in the salt bridge, only the transfer of electrons
between solutions. Because the question pertains to the salt bridge, and electrons do not migrate through the salt
bridge, no point was earned. In part (b)(i) the student correctly calculates the value of E
red
for the chromium half-
cell reaction and earned 1 point. In part (b)(ii) the student correctly balances the equation and earned 1 point. In
part (b)(iii) the student’s calculation of G is off by a factor of 10; thus, no point was earned.
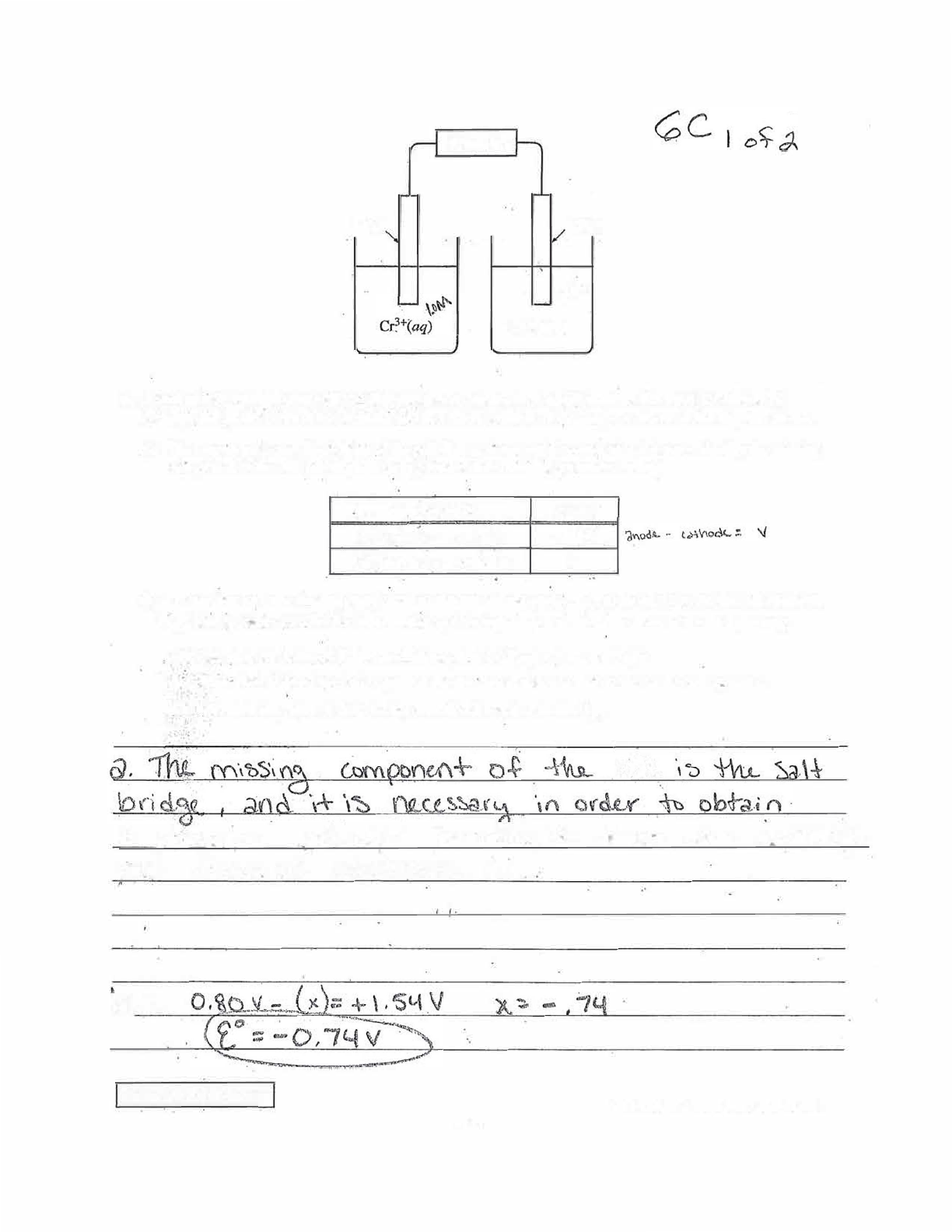
Sample: 6C
Score: 1
In part (a) the student erroneously states that the salt bridge “allows the exchange and flow of electrons,” rather
than ions, and does not address the migration of ions in the salt bridge, so no point was earned. In part (b)(i) the
student correctly calculates the value of E
red
for the chromium half-cell reaction, and 1 point was earned. In
part (b)(ii) the student misidentifies the oxidation and reduction half-reactions and also does not correctly balance
the species in the equation, so the point was not earned. In part (b)(iii) the student incorrectly identifies the
number of moles of electrons transferred in the reaction as n = 2, so no point was earned.
© 2018 The College Board.
Visit the College Board on the Web: www.collegeboard.org.
