
Centers for Disease Control and Prevention
Center for Preparedness and Response
Updates to COVID-19 Testing and Treatment for
the Current SARS-CoV-2 Variants
Clinician Outreach and Communication Activity (COCA) Call
Tuesday, January 24, 2023

Continuing Education
▪ Continuing Education is not offered for this webinar.

To Ask a Question
▪ Using the Zoom Webinar System
– Click on the “Q&A” button
– Type your question in the “Q&A” box
– Submit your question
▪ If you are a patient, please refer your question to your healthcare provider.
▪ If you are a member of the media, please direct your questions to
CDC Media Relations at 404-639-3286 or email media@cdc.gov.

cdc.gov/coronavirus
Updates to COVID-19 Testing and Treatment for the
Current SARS-CoV-2 Variants
Presenters:
Pragna Patel, MD MPH
CAPT, U.S. Public Health Service
Chief Medical Officer
COVID-19 Response
Centers for Disease Control and Prevention
Natalie Thornburg, PhD
Branch Chief of Lab Branch (Acting)
Coronavirus and Other Respiratory Viruses
Division (Proposed)
National Center for Immunization and
Respiratory Diseases
Centers for Disease Control and Prevention
Rajesh T. Gandhi, M.D.
Director, HIV Clinical Services and Education
Massachusetts General Hospital
Co-Director, Harvard Center for AIDS Research
Professor of Medicine, Harvard Medical School

cdc.gov/coronavirus
Update on COVID-19 Epidemiology
Pragna Patel, MD, MPH
CAPT, U.S. Public Health Service
Chief Medical Officer
COVID-19 Response
Centers for Disease Control and Prevention
Clinician Outreach and Communication Activity
January 24, 2023

Question
• How many COVID-19 cases have been reported so far, in
your opinion?

Answer
• The correct answer is 101,873,730.
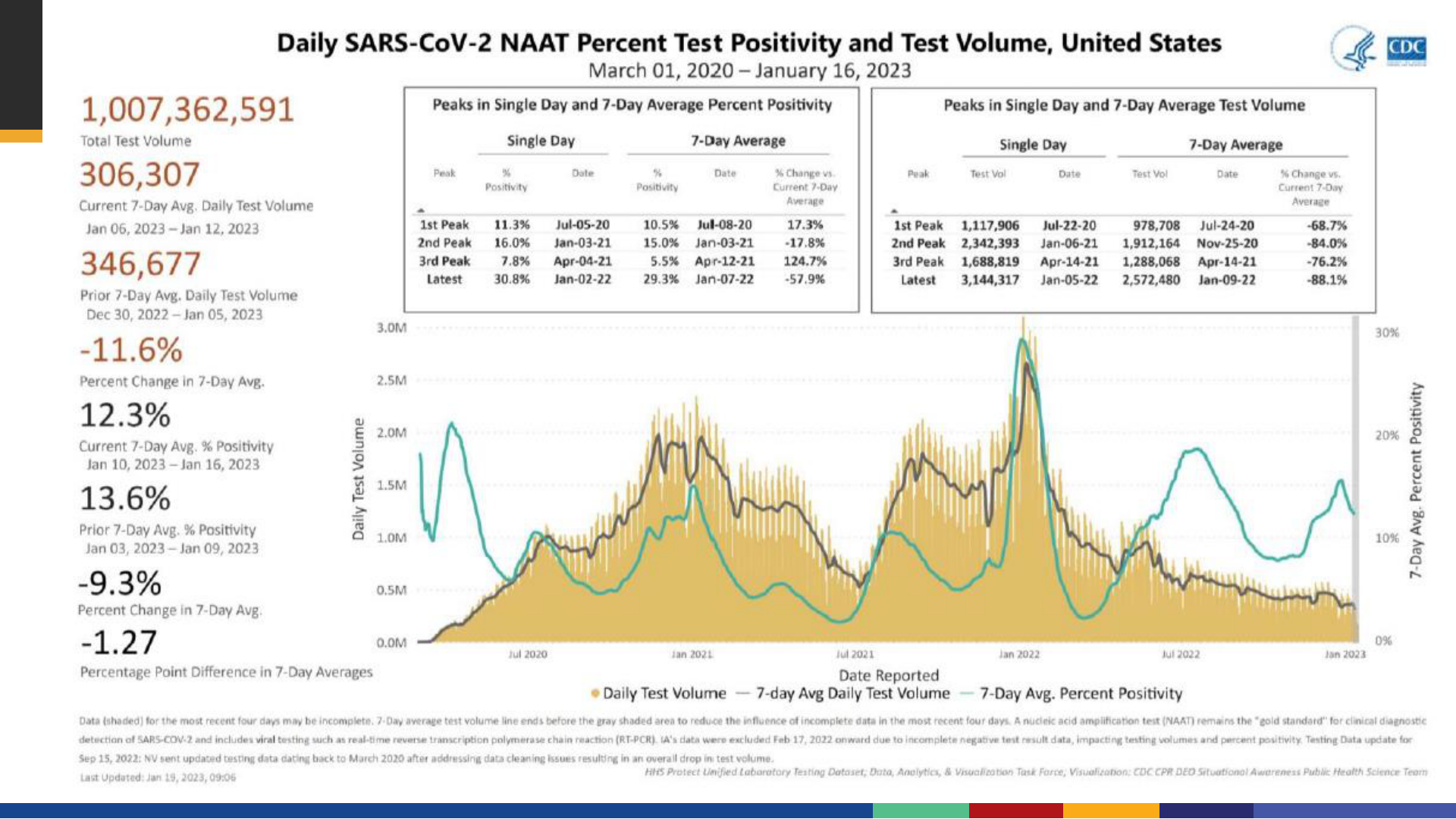

New Admissions of Patients with COVID-19 in the United States

Daily Trends in COVID-19 Deaths in the United States

cdc.gov/coronavirus
Diagnostic Testing Algorithm
Natalie Thornburg, PhD
Branch Chief of Lab Branch (Acting)
Coronavirus and Other Respiratory Viruses Division (Proposed)
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention

Question
▪ A patient has had COVID-19 like symptoms for 3 days and tests negative on a home-
based antigen test. Does the patient need any further follow up or is it safe to say
they are not infected with SARS-CoV-2?

Answer
▪ The patient needs further follow up. They should either seek testing with a NAAT or
1-2 more times with home-based antigen tests to say it’s safe they are not infected
with SARS-CoV-2. They should also consider contacting their health care provider to
consider alternative diagnoses.

Overview
▪ When to test
▪ Types of tests
▪ How to interpret tests
▪ Current landscape of SARS-CoV-2 genomics
▪ Performance of current tests with currently circulating viruses

Diagnostic tests are for symptomatic and exposed
persons
▪ Diagnostic tests are used when someone is:
– Symptomatic
– Known exposure to someone with SARS-CoV-2
▪ Screening tests are performed in specific environments on
asymptomatic people
– High risk settings (such as nursing homes or in health care settings)
– Before events or travel

Diagnostic test timing
▪ If symptomatic, patients should test immediately
– Limit exposure to others
– Starting treatment as early as possible for high risk
▪ If asymptomatic and known exposure, test at least 5 days
after exposure
– Wear a high-quality mask when around others inside the home or in
public for 10 days after exposure
– The incubation period of SARS-CoV-2 is about 3-5 days, and it may take
you that long to test positive
https://ww w.cdc.gov/coronav irus/2019-ncov/your-healt h/if-
you-were-exposed.html

Diagnostic tests are based on nucleic acid or protein
▪ Nucleic acid amplification tests (NAAT)
– PCRs, LAMP, CRISPR
– Often lab-based
– Highly sensitive and specific
– Patients often test positive for extended period of time, well beyond
infectiousness period
▪ Rapid antigen tests
– Detect viral protein
– May be POC (point-of-care) or at home
– Less sensitive than NAATs
– Virus must have replicated enough for protein to be detected
– Delayed positivity

If a patient tests negative by Rapid Antigen Test (RAT)
▪ FDA recommends
– If symptomatic, test at least twice 48 hours apart. A third test might be needed
if the patient is concerned they have COVID-19.
– If asymptomatic, but believe they have been exposed, test with RAT at least 3
times, each 48 hours apart to be considered truly negative
▪ Consider reflex testing to NAAT
– If NAAT is negative, consider alternative diagnoses such as flu, RSV, or strep throat

Summary
▪ For Symptomatic patients who haven’t had a recent infection, should test using
either RAT or NAAT as soon as possible
– If positive, they should isolate and consider treatment
– If negative by RAT, they should retest one-to-two more times per FDA
guidance, or seek testing with a NAAT
– If symptomatic patient tests negative at least 3 times by RAT or once by NAAT,
alternative diagnoses should be considered
▪ If a patient has had a recent infection and has new symptoms, use RATs, though
multiple negative tests may be needed.

COVID-19 Outpatient Treatment Updates
January 24, 2023
Rajesh T. Gandhi, MD
Director, HIV Clinical Services and Education, Massachusetts General Hospital
Co-Director, Harvard University Center for AIDS Research
Disclosures (past 2 years):
Member, NIH & Infectious Diseases Society of America COVID-19 Treatment Guidelines Panels;
Recommendations in this talk are my own and not necessarily those of the Panels
Acknowledgments: Arthur Kim, Jon Li, Courtney Tern

Case
• 62 yo woman presenting with 2 days of fever, cough, myalgias. SARS
CoV-2 rapid antigen test positive
• Oxygen saturation >95%
• History of HIV (CD4 cell count 350; HIV RNA undetectable), pulmonary
hypertension
• Medications: bictegravir/FTC/TAF; tadalafil 40 mg daily
• Received 2 doses of mRNA COVID-19 vaccine in 2021; has not received
any booster doses
• Would you treat? If so, with what?

COVID-19 Risk Continuum

SARS CoV-2 Antivirals
Anti-spike monoclonal
antibodies, including
bebtelovimab:
Not active against most
circulating SARS CoV-2 variants
Protease inhibitor:
Nirmatrelvir/ritonavir
(Paxlovid)
Molnupiravir
(Lagevrio)
Remdesivir
(Veklury)
Modified from https://www.science.org/doi/epdf/10.1126/science.acx9605

Omicron variants resistant to
Bebtelovimab (Beb)
Most prevalent variants
• XBB.1.5: 49.1%
• BQ.1.1: 26.9%
• BQ.1: 13.3%
• XBB: 3.3%
Jan 21, 2023: XBB.1.5, BQ.1.1,
BQ.1, XBB: vast majority of US
isolates
Omicron Beb
BQ.1, 1.1
XBB,
XBB.1.5
Modified from slide
by Dr Jon Li
Nov 30, 2022:

Slide 33
New Omicron variants resistant to
tixagevimab/cilgavimab
Jan 21, 2023: about 94% of US
variants anticipated to be resistant
to tixagevimab/cilgavimab
Omicron Tixa/cil
XBB,
XBB.1.5
BQ.1, 1.1
BF.7

National Institute of Health COVID-19
Treatment Guidelines
▪ Tixagevimab/cilgavimab unlikely to be effective in preventing COVID-19 for
vast majority because of high prevalence of resistant Omicron subvariants
▪ Given lack of other PrEP options, clinicians could still administer tixagevimab/
cilgavimab after considering individual’s risks and regional prevalence of
resistant subvariants
▪ Immunocompromised individuals who receive tixagivimab/cilgavimab should
be counseled to continue measures to avoid infection (including keeping up
to date with vaccination) and to seek testing and treatment if symptoms of
COVID-19 develop
https://www.covid19treatmentguidelines.nih.
gov/therapies/statement-on-evusheld/

Small molecule antivirals anticipated to be active against new variants
1)
Nirmatrelvir/r
2) Remdesivir
3)
Molnupiravir
Efficacy
(
hospitaliza-
tion
/death in
unvaccinated,
high risk
)
•Relative risk reduction:
88% (EPIC
-HR)
•Absolute risk: 6.3%→0.8%
•NNT: 18
•Relative risk reduction: 87%
(PINETREE)
•Absolute risk: 5.3%→0.7%
•NNT: 22
•Relative risk reduction: 30%
(
MOVe-OUT)
•Absolute risk: 9.7%→6.8%
•NNT: 35
Pros
•
Highly efficacious
•
Oral regimen
•
Ritonavir studied (safe) in
pregnancy
•
Highly efficacious
•
Studied in pregnancy
•
Few/no drug interactions
•
Oral regimen
•
Not anticipated to have drug
interactions
Cons
•
Drug drug interactions
•
Requires IV infusion on 3
consecutive days
•
Lower efficacy
•
Concern: mutagenicity
•
Not recommended in
pregnancy/children
Modified from Table in Gandhi RT, Malani P, del Rio C, JAMA, Jan 14, 2022

Should Vaccinated People be Treated?
Treat
Do Not Treat

Nirmatrelvir/r in People with Previous Immunity
• Retrospective cohort study in Israel
• N/r (n=3902); No N/r (n=105,352)
• ~80% had previous immunity
(vaccination, prior infection, both)
• ≥65 y: hospitalization less likely in treated
group (hazard ratio, 0.27). Benefit
regardless of previous immunity status.
• Patients aged 40–64, hospitalizations
similar in treated and untreated groups
Age ≥ 65 y
Age 40-64 y
Arbel R, NEJM, 2022

Nirmatrelvir/Ritonavir for Early COVID-19 in Large
US Health System
• 44,551 outpatients aged 50 years
or older with COVID-19
• 90% with ≥3 vaccine doses
• Hospitalization/death: 0.55%
(NMV/r) vs. 0.97% (no NMV/r)
• NMV/r recipients: lower risk for
hospitalization (adjusted RR=0.60)
and death (adjusted RR=0.29)
Dryden-Peterson S, Ann Intern Med, 2022

Nirmatrelvir/ritonavir: Drug Drug Interactions
• Ritonavir inhibits CYP3A during treatment (5 days) and for additional 2-3 days after
treatment completed
• Some medicines should not be coadministered, eg rivaroxaban, amiodarone, rifampin,
tadalafil (for pulmonary hypertension)
• Others may need to be held or markedly dose reduced, eg calcineurin inhibitors
• Other medications may be temporarily stopped: eg, atorvastatin, rosuvastatin
• Useful resources:
• NIH COVID-19 Treatment Guidelines
• IDSA Management of Drug Interactions: Resource for Clinicians
• Univ. of Liverpool COVID-19 Drug Interaction Checker
https://www.covid19treatmentguidelines.nih.gov/
https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-
management/management-of-drug-interactions-with-nirmatrelvirritonavir-paxlovid/
https://www.covid19-druginteractions.org/

Molnupiravir (MOV) in Vaccinated Adults: PANORAMIC
• Open-label, randomized controlled trial in
UK, Dec 2021 to April 2022
• ≈25,000 non-hospitalized adults with COVID
and symptoms for ≤5 days
• MOV + usual care vs. usual care
• Aged ≥50 y or ≥18 y with high-risk
comorbidity
• 94% received ≥3 COVID vaccine doses
• Hospitalization/death: 1% in both groups
• Time to self-reported recovery: 9 days
(MOV) vs. 15 days (usual care)
Butler CC, Lancet, 2022

Should Vaccinated People Be Treated? My Take
• Gradient of benefit: higher risk patients likely to derive more benefit
• Recommend treatment for older people, regardless of vaccination status
• For younger people who are vaccinated/boosted, recommend treatment
if they have conditions that confer substantial risk, including obesity,
heart or lung disease, immunosuppression, other high-risk conditions
• Not yet known whether early treatment ameliorates post-acute sequelae
of SARS CoV-2 (PASC) – important research and knowledge gap

VV116 vs Nirmatrelvir-Ritonavir (NMV/r)
• VV116: oral remdesivir analogue
• Phase 3, observer-blinded, randomized trial
during Omicron outbreak in China
• 771 adults with mild to moderate COVID-19 and
high risk of progression to severe disease
• About 75% fully vaccinated or boosted
• Randomized: VV116 or NMV/r for 5 days
• Time to sustained clinical recovery: VV116 non-
inferior to NMV/r; median time to symptom
resolution was 7 days in both groups
• No deaths or progression to severe disease
Cao Z, NEJM, 2022
Sustained clinical recovery
(alleviation of symptoms for two consecutive days)

Back to the Case
• 62 yo woman presenting with 2 days of fever, cough, myalgias. SARS
CoV-2 rapid antigen test positive.
• Oxygen saturation >95%
• HIV (CD4 cell count 350; HIV RNA undetectable), pulmonary
hypertension. Medications: bictegravir/FTC/TAF; tadalafil 40 mg daily
• 2 doses of mRNA COVID-19 vaccine in 2021; has not been boosted
• Treatment recommended. Because NMV/r cannot be given with her
pulmonary hypertension medicine (tadalafil), she received iv remdesivir
with rapid clinical improvement

Question
• The patient is an 85-year-old male, and up to date on his COVID-19 vaccinations.
• Has hyperlipidemia which is controlled with statin treatment and has a family
history of heart disease.
• Presents with a positive SARS-CoV-2 at home antigen test after developing mild
symptoms.
• Denies fever and reports only cough.
• Has had SARS-COV-2 in the past and was treated with ritonavir-boosted
nirmatrelvir, tolerated the metallic taste, and responded well with recovery in 2-3
days.
• Received his bivalent booster one week before presentation to your clinic.
• Should he receive COVID-19 treatment at this time? Why or why not? What is
important to consider in your decision to treat?

Answer
• Treatment is recommended especially if his last bout of Covid and his last vaccine
booster prior to the bivalent vaccine were more than 3-6 months ago and if he is
within 5 days of symptom onset.

To Ask a Question
▪ Using the Zoom Webinar System
– Click on the “Q&A” button
– Type your question in the “Q&A” box
– Submit your question
▪ If you are a patient, please refer your question to your healthcare provider.
▪ If you are a member of the media, please direct your questions to
CDC Media Relations at 404-639-3286 or email media@cdc.gov.

Today’s COCA Call Will Be Available On-Demand
▪ When: A few hours after the live call
▪ What: Video recording
▪ Where: On the COCA Call webpage at:
https://www.emergency.cdc.gov/coca/calls/2022/callinfo_012423.asp
▪ Subscribe to receive notifications about upcoming COCA Calls and other
COCA products and services at emergency.cdc.gov/coca/subscribe.asp

COCA Products & Services
COCA Call Announcements contain all information
subscribers need to participate in COCA Calls. COCA
Calls are held as needed.
Monthly newsletter that provides information on CDC
training opportunities, conference and training
resources, the COCA Partner Spotlight, and the
Clinician Corner.
As-needed messages that provide specific, immediate
action clinicians should take. Contains comprehensive
CDC guidance so clinicians can easily follow
recommended actions.

Join Us On Facebook!










